AP Chemistry Unit 3 MCQ: A Comprehensive Guide sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This guide delves into the fundamental concepts of chemical kinetics, equilibrium, and thermodynamics, providing a thorough understanding of the intricacies of AP Chemistry Unit 3.
Through a comprehensive analysis of MCQ structure and format, readers will gain valuable insights into the types of questions commonly encountered and the strategies for answering them effectively. The guide also elaborates on problem-solving techniques specific to AP Chemistry Unit 3, providing examples to illustrate their application in solving MCQs.
Concept Review
AP Chemistry Unit 3 delves into the intricate world of chemical kinetics, equilibrium, and thermodynamics, providing a comprehensive understanding of the dynamics and energy transformations that govern chemical reactions.
Chemical kinetics explores the rates of reactions, delving into the factors that influence the speed and mechanisms by which reactants transform into products. Equilibrium, on the other hand, focuses on the dynamic balance between opposing reactions, where the concentrations of reactants and products remain constant over time.
Thermodynamics, the study of energy changes, provides insights into the spontaneity and efficiency of chemical reactions.
Chemical Kinetics
Chemical kinetics unravels the intricate mechanisms and factors that govern the rates of chemical reactions. It investigates the relationship between the concentrations of reactants, temperature, and the presence of catalysts, exploring how these variables influence the speed and pathways of reactions.
- Reaction Rates: Understanding the factors that affect the rate of a reaction, such as concentration, temperature, and surface area.
- Reaction Mechanisms: Exploring the step-by-step pathways through which reactants transform into products, including the identification of intermediates and transition states.
- Catalysis: Investigating the role of catalysts in enhancing reaction rates, examining different types of catalysts and their mechanisms of action.
Equilibrium
Equilibrium is a dynamic state in which the forward and reverse reactions of a chemical process occur at equal rates, resulting in no net change in the concentrations of reactants and products. This concept plays a crucial role in understanding chemical systems and predicting their behavior.
- Equilibrium Constant: Determining the equilibrium constant, a quantitative measure of the extent to which a reaction proceeds towards completion.
- Le Chatelier’s Principle: Predicting the shifts in equilibrium position in response to changes in temperature, pressure, or concentration.
- Applications of Equilibrium: Exploring the practical applications of equilibrium principles in various fields, such as industrial processes and environmental chemistry.
Thermodynamics
Thermodynamics delves into the energy changes associated with chemical reactions, providing insights into the spontaneity and efficiency of these processes. It investigates the concepts of enthalpy, entropy, and free energy, which govern the direction and extent of reactions.
Conquering AP Chemistry Unit 3 MCQs requires a solid foundation. While studying for this, you might wonder about the pass rate for the PSIA Level 1 exam. If you’re curious, you can check out the psia level 1 pass rate . Returning to our topic, mastering AP Chemistry Unit 3 MCQs is crucial for success in the exam.
- Enthalpy: Understanding the heat changes that accompany chemical reactions, including exothermic and endothermic processes.
- Entropy: Exploring the concept of entropy as a measure of disorder and its role in determining the spontaneity of reactions.
- Free Energy: Investigating the Gibbs free energy, which combines enthalpy and entropy to predict the spontaneity and equilibrium position of reactions.
MCQ Analysis: Ap Chemistry Unit 3 Mcq
AP Chemistry Unit 3 MCQs assess your understanding of equilibrium, acids and bases, and thermodynamics.
These MCQs typically consist of multiple-choice questions, with four or five answer choices. The questions may be straightforward or require you to apply multiple concepts to solve them effectively.
Types of Questions
- Conceptual questions:Test your understanding of fundamental concepts and theories.
- Application questions:Require you to apply concepts to solve problems or analyze scenarios.
- Calculation questions:Involve numerical calculations and require you to apply equations and formulas.
Strategies for Answering MCQs
- Read the question carefully:Identify the main concept being tested and the specific information you need to answer it.
- Eliminate incorrect answers:Cross out answer choices that are clearly incorrect or irrelevant.
- Consider the context:Use the information provided in the question or passage to help you choose the correct answer.
- Estimate the answer:If you’re unsure, try to estimate the answer based on your knowledge of the topic.
- Guess strategically:If you’re running out of time, guess based on the probability of each answer choice being correct.
Problem-Solving Techniques
In AP Chemistry Unit 3, you’ll encounter MCQs that require you to apply specific problem-solving techniques. These techniques are designed to help you analyze the problem, identify the relevant concepts, and develop a solution.
One key technique is dimensional analysis. This involves converting units between different systems to ensure that the units in your answer match the units in the question. For example, if a question asks you to calculate the molarity of a solution, you need to convert the volume from milliliters to liters and the mass from grams to moles before you can plug the values into the formula.
Dimensional Analysis, Ap chemistry unit 3 mcq
Dimensional analysis is a powerful tool that can help you solve a variety of chemistry problems. It involves converting units between different systems to ensure that the units in your answer match the units in the question.
- To use dimensional analysis, start by identifying the units in the question.
- Then, convert the units in your answer to match the units in the question.
- You can use conversion factors to help you convert between units.
For example, if a question asks you to calculate the molarity of a solution, you need to convert the volume from milliliters to liters and the mass from grams to moles before you can plug the values into the formula.
- 1 L = 1000 mL
- 1 mol = 6.022 x 10 23atoms
Once you have converted the units, you can plug the values into the formula and solve for the unknown.
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. It is used to calculate the amount of reactants and products that are involved in a reaction.
- To use stoichiometry, you need to know the balanced chemical equation for the reaction.
- Once you have the balanced chemical equation, you can use the mole ratios to calculate the amount of reactants and products that are involved in the reaction.
For example, if you want to calculate the mass of sodium chloride that is produced when 10.0 g of sodium reacts with 10.0 g of chlorine, you would use the following steps:
- Write the balanced chemical equation for the reaction: 2 Na + Cl2→ 2 NaCl
- Calculate the mole ratio of sodium to sodium chloride: 2 mol Na / 2 mol NaCl = 1 mol Na / 1 mol NaCl
- Calculate the mole ratio of chlorine to sodium chloride: 1 mol Cl 2/ 2 mol NaCl = 1 mol Cl 2/ 2 mol NaCl
- Convert the mass of sodium to moles: 10.0 g Na / 22.99 g/mol = 0.435 mol Na
- Convert the mass of chlorine to moles: 10.0 g Cl 2/ 70.90 g/mol = 0.141 mol Cl 2
- Use the mole ratios to calculate the moles of sodium chloride that is produced: 0.435 mol Na x 1 mol NaCl / 1 mol Na = 0.435 mol NaCl
- Convert the moles of sodium chloride to mass: 0.435 mol NaCl x 58.44 g/mol = 25.4 g NaCl
Therefore, the mass of sodium chloride that is produced when 10.0 g of sodium reacts with 10.0 g of chlorine is 25.4 g.
Common Mistakes and Pitfalls
Mistakes and pitfalls are common when answering AP Chemistry Unit 3 MCQs. Understanding and avoiding these errors can significantly improve accuracy and overall performance.
One common pitfall is misinterpreting the question. Students may rush to answer without carefully reading and understanding the question’s intent. It’s crucial to take time to identify the main concept being tested and any specific details or conditions mentioned.
Misreading the Question
- Read the question thoroughly and identify the key concept.
- Pay attention to specific details and conditions mentioned in the question.
- Rephrase the question in your own words to ensure understanding.
Test-Taking Strategies
Maximizing your performance on the AP Chemistry Unit 3 exam requires effective test-taking strategies. Mastering time management, question selection, and educated guessing techniques can significantly enhance your score.
Time Management
- Prioritize questions based on difficulty and familiarity. Start with easier questions to build confidence and save time for more challenging ones.
- Allocate time wisely. Dedicate more time to questions worth more points and avoid spending excessive time on difficult questions.
- Pace yourself throughout the exam. Don’t get bogged down on any particular question. Move on and return to it later if time permits.
Question Selection
- Read questions carefully and identify key concepts. Determine what type of question it is (multiple choice, free response, etc.) and what specific information it’s asking for.
- Eliminate obviously incorrect answer choices to narrow down your options.
- Guess intelligently if you’re unsure. Eliminate choices that seem unlikely or that you can rule out based on your knowledge.
Guessing Techniques
- Educated Guessing:If you have some knowledge about the topic, use logical reasoning and process of elimination to make an educated guess.
- Guess by Pattern:If you notice a pattern in the answer choices (e.g., alternating between A and B), you can guess based on that pattern.
- Guess by Similarity:If you recognize a similar question or concept from your studies, use that knowledge to guide your guess.
Practice and Review
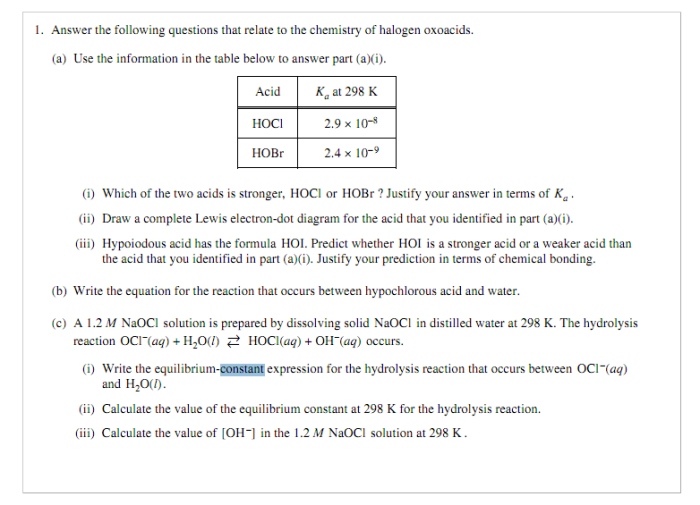
Mastering AP Chemistry requires consistent practice and a thorough review of key concepts. This section provides guidance on organizing practice MCQs and creating a comprehensive review guide to enhance your preparation.
By implementing these strategies, you can effectively reinforce your understanding of the material, identify areas for improvement, and boost your confidence for the exam.
Practice MCQs
Organize practice MCQs into different difficulty levels to cater to your progress and understanding. Start with easier questions to build a strong foundation and gradually increase the complexity to challenge yourself.
Seek practice questions from various sources, including textbooks, online platforms, and past AP exams. This diversity exposes you to different question formats and perspectives, broadening your understanding of the subject.
Review Guide
Create a comprehensive review guide that condenses key concepts, formulas, and problem-solving techniques into a concise and accessible format.
Include clear explanations, worked examples, and practice problems to reinforce your understanding. This guide should serve as a valuable resource for quick revision and targeted review before the exam.
Key Questions Answered
What is the best way to prepare for AP Chemistry Unit 3 MCQs?
By understanding the fundamental concepts, practicing regularly, and utilizing effective test-taking strategies.
What are the common types of questions encountered in AP Chemistry Unit 3 MCQs?
Multiple-choice questions, short answer questions, and free-response questions.
How can I avoid common mistakes when answering AP Chemistry Unit 3 MCQs?
By carefully reading the questions, understanding the concepts, and eliminating incorrect answer choices.