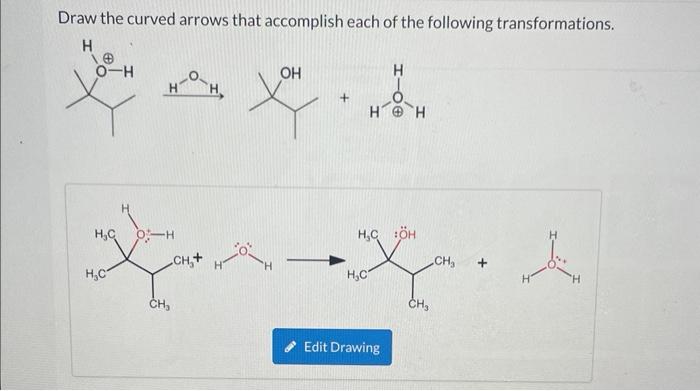
Identify the expected major product of the following diels-alder reaction – The Diels-Alder reaction is a powerful tool for the construction of cyclic compounds. It is a [4+2] cycloaddition reaction between a conjugated diene and a dienophile. The reaction proceeds through a concerted mechanism, and the regio- and stereoselectivity of the reaction are determined by the electronic properties of the reactants.
In this article, we will discuss the factors that affect the regio- and stereoselectivity of the Diels-Alder reaction and how to identify the expected major product of a given reaction.
The Diels-Alder Reaction
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile. The reaction is named after Otto Diels and Kurt Alder, who first reported the reaction in 1928.The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds.
The reaction is typically carried out in a solvent such as dichloromethane or benzene. The diene and dienophile are heated together in the presence of a Lewis acid catalyst, such as aluminum chloride or tin(IV) chloride.The mechanism of the Diels-Alder reaction is a concerted process that involves the formation of a new carbon-carbon bond between the diene and the dienophile.
The reaction proceeds through a transition state that is stabilized by the Lewis acid catalyst.The stereochemistry of the Diels-Alder reaction is determined by the orientation of the diene and the dienophile. The reaction can proceed through either an endo or exo transition state.
The endo transition state leads to the formation of a cis-substituted cyclohexene, while the exo transition state leads to the formation of a trans-substituted cyclohexene.The regioselectivity of the Diels-Alder reaction is determined by the electronic properties of the diene and the dienophile.
The diene typically reacts with the dienophile in a 1,4-fashion, but 1,2- and 1,3-additions are also possible.
Identify the Expected Major Product

The expected major product of a Diels-Alder reaction can be determined by considering the regio- and stereoselectivity of the reaction.The regioselectivity of the reaction is determined by the electronic properties of the diene and the dienophile. The diene typically reacts with the dienophile in a 1,4-fashion, but 1,2- and 1,3-additions are also possible.
The regioselectivity of the reaction can be predicted using the following rules:
- The diene will react with the dienophile in a way that maximizes the number of new carbon-carbon bonds formed.
- The diene will react with the dienophile in a way that minimizes the steric hindrance between the two molecules.
The stereoselectivity of the reaction is determined by the orientation of the diene and the dienophile. The reaction can proceed through either an endo or exo transition state. The endo transition state leads to the formation of a cis-substituted cyclohexene, while the exo transition state leads to the formation of a trans-substituted cyclohexene.
The stereoselectivity of the reaction can be predicted using the following rules:
- The reaction will proceed through the endo transition state if the diene and the dienophile are both electron-rich.
- The reaction will proceed through the exo transition state if the diene and the dienophile are both electron-poor.
Examples
The Diels-Alder reaction is a versatile reaction that can be used to synthesize a wide variety of cyclic compounds. Some examples of Diels-Alder reactions include:
- The reaction of 1,3-butadiene with maleic anhydride to form 4-cyclohexene-1,2-dicarboxylic anhydride
- The reaction of 1,3-cyclohexadiene with ethylene to form bicyclo[2.2.1]hept-2-ene
- The reaction of 1,3-cyclopentadiene with acrylic acid to form 3-cyclopentene-1-carboxylic acid
Applications
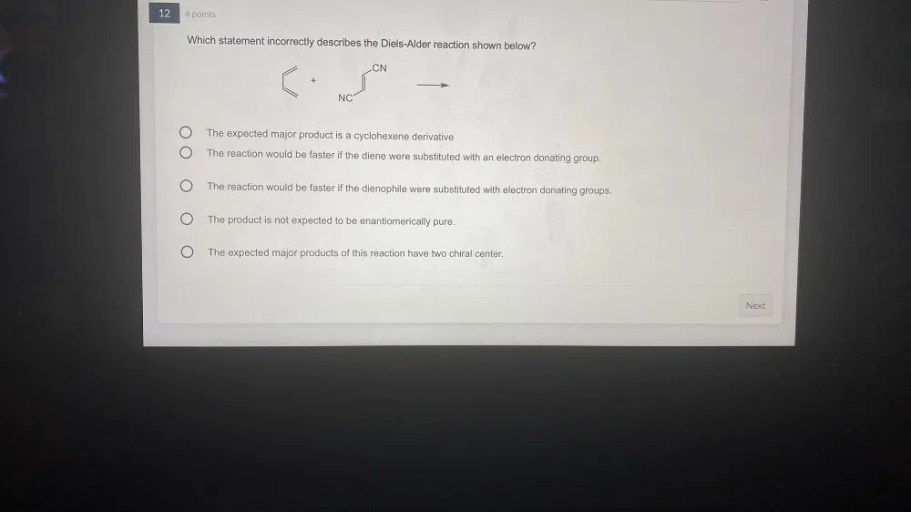
The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds. The reaction is used in the synthesis of a wide variety of natural products and pharmaceuticals. Some examples of the applications of the Diels-Alder reaction include:
- The synthesis of steroids
- The synthesis of alkaloids
- The synthesis of antibiotics
- The synthesis of vitamins
Expert Answers: Identify The Expected Major Product Of The Following Diels-alder Reaction
What is the Diels-Alder reaction?
The Diels-Alder reaction is a [4+2] cycloaddition reaction between a conjugated diene and a dienophile.
What are the factors that affect the regio- and stereoselectivity of the Diels-Alder reaction?
The regio- and stereoselectivity of the Diels-Alder reaction are determined by the electronic properties of the reactants.
How can I identify the expected major product of a given Diels-Alder reaction?
To identify the expected major product of a given Diels-Alder reaction, you need to consider the regio- and stereoselectivity of the reaction.